Every schoolchild faces physics lessons withsuch a concept as "specific heat". In most cases, people forget the school definition, and often do not understand the meaning of the term. In technical universities, most students will sooner or later face specific heat capacity. Perhaps in the framework of studying physics, or maybe someone will have such a discipline as "heat engineering" or "technical thermodynamics." In this case, you will have to remember the school curriculum. So, below is the definition, examples, values for some substances.
Definition
Specific heat is physicala quantity that characterizes how much heat must be supplied to a unit of a substance or taken from a unit of a substance so that its temperature changes by one degree. It is important to cancel that it does not matter, degrees Celsius, Kelvin and Fahrenheit, the main thing - the change in temperature by one.
Удельная теплоемкость имеет свою единицу measurements - in the international system of units (SI) - Joule divided by the product of a kilogram and degree Kelvin, J / (kg · K); the non-system unit is the ratio of the calorie to the product of the kilogram and degree Celsius, cal / (kg · ° C). This value is most often denoted by the letter c or C, sometimes indices are used. For example, if the pressure is constant, then the index is p, and if the volume is constant, then v.
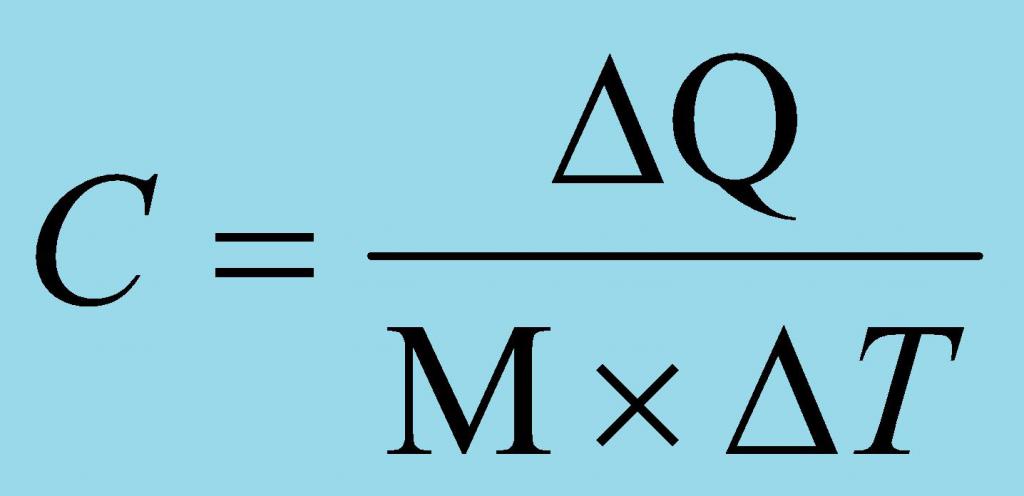
Variations of definition
There are several definitions possible.discussed physical quantity. In addition to the above, a definition is considered acceptable, which states that the specific heat capacity is the ratio of the heat capacity of a substance to its mass. In this case, it is necessary to clearly understand what "heat capacity" is. So, the heat capacity is the physical quantity, which shows how much heat must be brought to the body (substance) or divert in order to change its temperature value by one. The specific heat of the mass of a substance greater than a kilogram is determined similarly as for a single value.
Some examples and meanings for different substances.

Экспериментально выяснено, что для разных веществ this value is different. For example, the specific heat capacity of water is 4.187 kJ / (kg · K). The largest value of this physical quantity in hydrogen is 14.300 kJ / (kg · K), the smallest in gold is 0.129 kJ / (kg · K). If you need a value for a particular substance, then you need to take a directory and find the appropriate tables, and in them - the values of interest. However, modern technologies make it possible to speed up the search process at times — it’s enough on any phone that has the option to enter the world wide web, type the question of interest in the search line, start a search and search for an answer based on the results. In most cases it is necessary to follow the first link. However, sometimes it’s not at all necessary to go anywhere else - the brief description of the information shows the answer to the question.

The most common substances for which heat capacity is sought, including specific heat, are:
- air (dry) - 1,005 kJ / (kg · K),
- aluminum - 0.930 kJ / (kg · K),
- copper - 0.385 kJ / (kg · K),
- ethanol - 2.460 kJ / (kg · K),
- iron - 0.444 kJ / (kg · K),
- mercury - 0,139 kJ / (kg · K),
- oxygen - 0.920 kJ / (kg · K),
- wood - 1,700 kJ / (kg · K),
- sand - 0.835 kJ / (kg · K).












