Hydrocarbons - the simplest class of organicsubstances, their molecules have atoms of only two chemical elements - carbon and hydrogen. Most of the classes of organic compounds are obtained from various hydrocarbons using chemical synthesis methods.
Hydrocarbons are divided into two subclasses -acyclic and cyclic. The acyclic hydrocarbons, or fatty hydrocarbons, or aliphatic hydrocarbons, include the following groups: saturated hydrocarbons (alkanes), unsaturated (alkenes, alkynes, dienes), acyclic terpenes. Cyclic hydrocarbons are represented by groups of cycloparaffins, arenes and cyclic terpenes. Sometimes terpenes are classified as bioorganic chemistry objects.
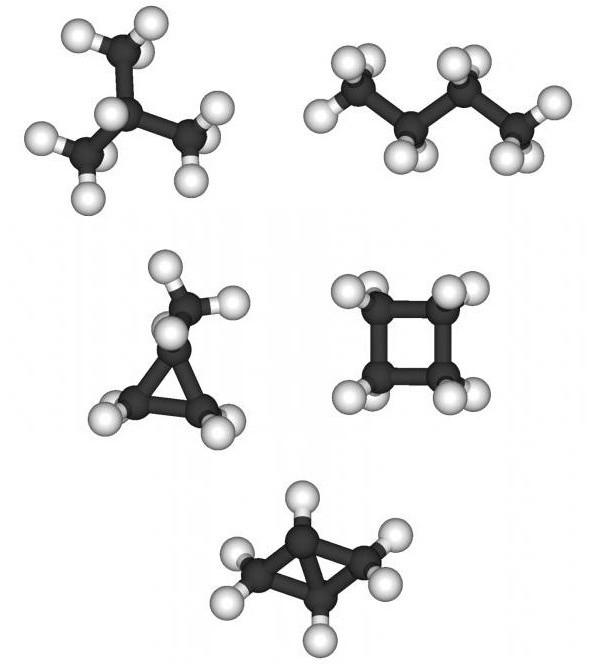
Saturated hydrocarbons (alkanes) - compoundscarbon and hydrogen, in the molecules of which all valences remaining after the carbon atoms are simply bonded to each other, are saturated with hydrogen atoms. All alkanes can be considered as derivatives or homologs of methane. If one atom of hydrogen is taken away from methane, which has the molecular formula CH4, a particle is formed - the radical CH3. Due to the fact that in the molecule of organic matter the carbon is usually tetravalent, the combination of two such radicals causes the appearance of the second representative of the homologous series - ethane (C2H6). If one atom of hydrogen is taken away from ethane, an ethyl radical is formed, which, after combining with CH3, forms the third homolog — propane.
By analyzing the structural formula of propane, it is easyestablish that the composition of its molecules includes two types of carbon atoms - primary and secondary. Each primary carbon atom is bound to one carbon atom by one of its valence, and the secondary one by two valences with two carbon atoms. If a hydrogen atom is taken away from the primary carbon atom of propane, a primary propyl is formed, and a secondary propyl is formed from the secondary one. Adding to the primary or secondary propyl methyl causes the formation of structural species of the fourth homolog. Two compounds are formed - normal butane with a straight carbon chain and isobutane with a branched carbon chain.
Saturated hydrocarbons: structure
A typical representative of alkanes is methane.Molecular formula CH4. For alkanes, sigma bonds are characteristic. In a methane molecule, a carbon atom forms four covalent bonds at the expense of one s- and three p-orbitals, and each hydrogen atom at the expense of an s-orbital.
Saturated hydrocarbons: nomenclature and isomerism
When deriving structural homologous formulasa series of methane, starting with butane C4H10, we encounter the phenomenon of isomerism. For example, two individual compounds correspond to the molecular formula C4H10, C5H12 - three. In the future, the number of isomers with increasing number of carbon atoms in the alkane molecule increases. For example, five structural formulas and respectively individual substances correspond to molecular formula C6H14, C7H16-9, C8H18-18, C10H22 76, C12H26 355. The first four representatives of alkanes are gases, from the fifth to the twelfth - liquids, starting from the sixteenth - solids .
Chemical properties of saturated hydrocarbons
All saturated hydrocarbons are inert substances.This is due to the fact that in the molecules of alkanes the atoms of carbon and hydrogen are interconnected by sigma bonds, therefore, these compounds cannot add hydrogen atoms. Alkanes enter into radical halogenation, nitration, and cleavage reactions. In the process of halogenation, halogen atoms easily replace hydrogen atoms in the alkane molecule. When nitriding, the nitro group readily substitutes hydrogen in tertiary ones, and it is more difficult in the secondary and primary carbon atoms.
Limit and unsaturated hydrocarbons areraw materials for obtaining a variety of organic substances. By splitting off hydrogen atoms from saturated hydrocarbons, one can obtain unsaturated (alkenes, alkynes).