In chemistry there are groups of organic substances,differing in similar properties and united by a general formula that describes the pattern of the structural difference of each subsequent member of the group from the previous one. For example, a homologous series of alkanes, alkenes, alkynes or other groups. This nomenclature is of great importance for research, forecasting or practical application. For organic substances combined in a group, there are regular changes in chemical and physical properties, and all of them correlate with the change in molecular weight.
No less important are the rules describing how theproperties of substances in the transition from one group to another. To understand what a homological series is, one should consider specific examples. For any group of compounds, the increasing temperatures of melting (crystallization), boiling (condensation) and density with increasing molecular weight and number of carbon atoms in the molecule are characteristic.
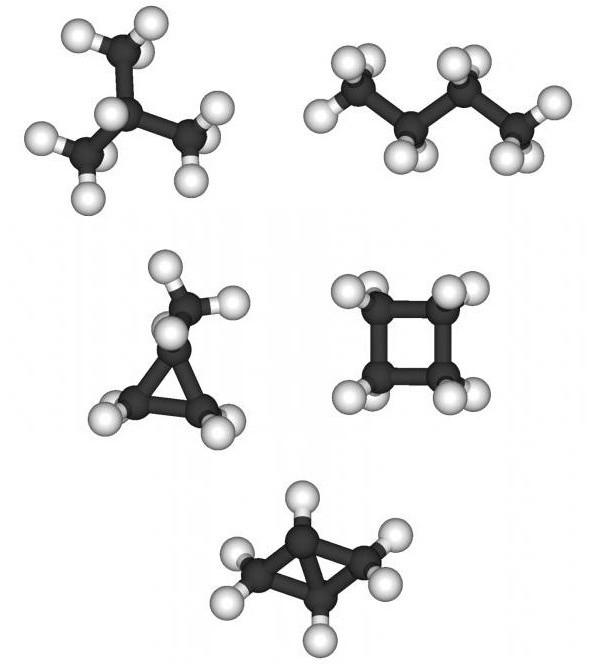
Limiting hydrocarbons are called saturated orparaffin; they are acyclic (no cycles) compounds of a normal or branched structure, the atoms in the molecules of which are connected by single bonds. The general formula has the form CnH2n + 2 and describes the homologous series of alkanes. The molecule of each next term increases in comparison with the previous one by one atom C and two atoms H. The saturated hydrocarbons include:
- methane;
- ethane;
- propane and so on.
Saturated hydrocarbons also includeCycloparaffins. This is a large group of organic compounds, the molecules of which are closed by rings. The homologous series of them has the formula CnH2n, starts with a chemical substance with three carbon atoms. Examples of Cycloparaffins:
- cyclopropane;
- cyclobutane;
- cyclopentane and so on
Unsaturated or unsaturated hydrocarbons alsoare acyclic. These include substances of normal and isostroeniya. The homologous series of alkenes has the general formula CnH2n. These compounds are distinguished by the presence of one double bond between two carbon atoms. If the previous series starts with a hydrocarbon with one carbon atom (methane), then this begins with a substance in which the molecule contains two carbon atoms. Examples of alkenes:
- ethen;
- propene;
- butene and so on.
Hydrocarbons, in the molecule of which two atomscarbon are linked by a triple bond, are even more unsaturated, otherwise they are called acetylene. They are joined by a homologous series of alkynes. It is described by the formula CnH2n-2 and begins with acetylene, in the formula of which there are two C atoms. Examples of alkynes:
- ethyn;
- propyne;
- butyn-1 and so on.
Unsaturated acyclic hydrocarbons, inmolecule of which there are two double bonds, are called dienic. They have the general formula CnH2n-2. Their homologous series begins with a hydrocarbon with three carbon atoms in the molecule. Double bonds can be conjugated (separated by a single bond), cumulated (located near neighboring atoms) or isolated (separated by several single bonds). Examples of dienes:
- 1,2-propadiene;
- 1,3-butadiene;
- isoprene and so on
A special group is made up of chemicalsa cyclic structure in whose molecule there is a benzene ring. The homologous series of the simplest aromatic hydrocarbons begins with a compound with six carbon atoms - benzene. Homologues of this series are formed by replacing one or several hydrogen atoms attached to the benzene ring with radicals. Thus, a number of substances are obtained: benzene, toluene, xylene. If there are two or more substituents in the molecule, then the presence of isomers for these substances is indicated. Other homologous series of aromatic compounds are formed from naphthalene, anthracene and other substances.
If there is a functional group in the hydrocarbon molecule, then such chemical compounds also form a homologous series.
- Ряд спиртов отличается наличием в молекуле hydroxyl group (-OH). For monohydric alcohols, one hydrogen atom in the acyclic hydrocarbon is replaced by a hydroxyl group; their formula: CnH2n + 1OH. There are also series of polyhydric alcohols.
- A number of phenols are characterized by the presence of a hydroxyl group (-OH) in the molecule, but it replaces hydrogen in the benzene ring.
- A number of aldehydes is distinguished by the presence in the molecule of a chemical compound of the carbonyl group (> C = O); The general formula for aldehydes is: R-CH = O.
- A number of ketones also distinguish the presence of a carbonyl group (> C = O), but if it is connected to one radical in aldehydes, then there are two ketones of hydrocarbon radicals. The ketone formula: R1-CO-R2.
- A number of carboxylic acids differ from other chemicals carboxyl group, which combines carbonyl and hydroxyl groups. The formula is RCOOH.
For each series, be it the homology seriesaldehydes, carboxylic (organic) acids, alcohols or other substances, their properties will largely be determined by the type of functional group and will naturally vary along with the increase in the molecular mass of the substance. Such a classification of a vast class of chemical compounds helps to understand nature and study their properties.