The simplest organic compounds are saturated and unsaturated hydrocarbons. These include substances of the class of alkanes, alkynes, alkenes.
Their formulas include hydrogen and carbon atoms in a specific sequence and quantity. They are often found in nature.
Determination of alkenes
Their other name is olefins or ethylene hydrocarbons. This is the name given to this class of compounds in the 18th century when an oily liquid was discovered - ethylene chloride.
Alkenes include substances consisting ofhydrogen and carbon elements. They refer to acyclic hydrocarbons. In their molecule there is a single double (unsaturated) bond connecting the two carbon atoms to each other.
Alkenes Formulas
Each class of compounds has its own chemical designation. In them, the symbols of the elements of the periodic system indicate the composition and structure of the bond of each substance.

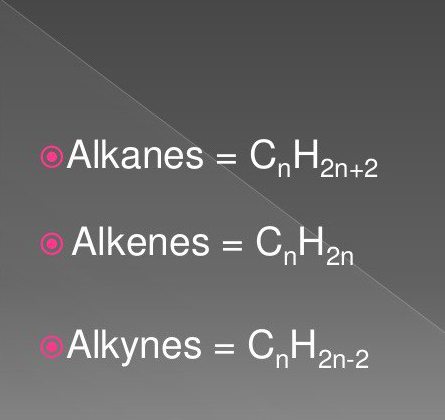
The general formula of alkenes is indicated as follows: CMr.X2nwhere the number n is greater than or equal to 2. When deciphering it, it is clear that for each carbon atom there are two hydrogen atoms.
Molecular formulas of alkenes from the homologous series are represented by the following structures: C2X4, C3X6, FROM4X8, C5X10, C6X12, C7X14, C8X16, C9X18, C10X20. It can be seen that each subsequent hydrocarbon contains one more carbon and two more hydrogen.
There is a graphic designation of the location and order of chemical compounds between atoms in a molecule, which shows the alkenes structural formula. With the help of valence dashes denotes the bond of carbon with hydrogen.
Alkenes structural formula can be depictedexpanded when all chemical elements and bonds are shown. With a shorter expression of olefins, the combination of carbon and hydrogen with valence dashes is not shown.
The skeletal formula means the simplest structure. A broken line depicts the basis of the molecule in which the carbon atoms are represented by its tips and ends, and hydrogen is indicated in units.
How the names of olefins are formed
Based on the systematic nomenclature, the formulaalkenes and their names make up the structure of alkanes belonging to saturated hydrocarbons. To do this, in the name of the latter the suffix -an is replaced by -ilen or -en. An example is the formation of butylene from butane, and pentene from pentane.
To indicate the position of the double bond relative to carbon atoms indicate the Arabic numeral at the end of the name.
The name of alkenes is based on the designationhydrocarbon with the longest chain, in which there is a double bond. For the beginning of the numbering of the chain, usually choose the end that is closest to the unsaturated compound of carbon atoms.
If the structural formula of alkenes hasbranches, then indicate the names of the radicals and their number, and before them put the numbers corresponding to the place in the carbon chain. Then follows the name of the hydrocarbon itself. After the numbers usually put a hyphen.
There are unsaturated radical branches. Their names can be trivial or are formed according to the rules of systematic nomenclature.
For example, HHC = CH- is referred to as ethenyl or vinyl.
Isomers
Alkene molecular formulas cannot indicate isomerism. However, for this class of substances, with the exception of the ethylene molecule, spatial modification is inherent.

Isomers of ethylene hydrocarbons can be on the carbon skeleton, on the position of the unsaturated bond, interclass or spatial.
The general formula of alkenes determines the amountcarbon and hydrogen atoms in the chain, but it does not show the presence and location of the double bond. An example is cyclopropane as an interclass isomer C3X6 (propylene). Other types of isomerism occur in C4X8 or butene.
The different position of the non-terminal relation is observed inbutene-1 or butene-2, in the first case the double compound is located near the first carbon atom, and in the second - in the middle of the chain. Isomerism on the carbon skeleton can be considered on the example of methylpropene (CH3-C (CH3) = H2) and isobutylene ((CH3) 2C = CH2).
Spatial modification is inherent in butene-2 intrans and cis position. In the first case, the side radicals are located above and below the main carbon chain with a double bond, in the second isomer the substituents are on the same side.
Characteristics of olefins
The general formula of alkenes determines the physical state of all members of this class. From ethylene to butylene (from C2 to C4), substances exist in gaseous form. So the colorless ethene has a sweetish smell, low solubility in water, the molecular weight is less than that of air.
Hydrocarbons of homologous gap from С are presented in liquid form.5 to C17. Starting with an alkene having 18 carbon atoms in the main chain, the physical state is transformed into a solid form.
All olefins are considered bad solubility inwater, but good in organic solvents, such as benzene or gasoline. Their molecular weight is less than that of water. An increase in the carbon chain leads to an increase in temperature indices during melting and boiling of these compounds.
Properties of olefins
The structural formula of alkenes indicates the presence ofthe skeleton of a double bond of π- and σ-compounds of two carbon atoms. This structure of the molecule determines its chemical properties. The bond-π is considered not very strong, which makes it possible to destroy it with the formation of the new two bonds-σ, which are obtained as a result of joining a pair of atoms. Unsaturated hydrocarbons are electron donors. They are involved in the processes of accession by electrophilic type.
An important chemical property of all alkenes isa halogenation process with isolation of compounds like dihalo derivatives. Halogen atoms are capable of attaching double bonds to carbon. An example is the bromination of propylene with the formation of 1,2-dibromopropane:
X2C = CH – CH3 + Br2 → BrCH2–CHBr – CH3.
This process of neutralizing color in bromine water by alkenes is considered qualitative evidence of the presence of a double bond.
The important reactions include the hydrogenation of olifinovwith the addition of a hydrogen molecule under the action of catalytic metals such as platinum, palladium or nickel. The result is a hydrocarbon with a saturated bond. Formulas of alkanes, alkenes are given below in the reaction of butene hydrogenation:
H3–H2–CH = CH2 + H2 Neither→ CH3–H2–H2–H3.
The process of attaching a hydrogen halide molecule to olefins is called
hydrohalogenation, passing according to the ruleopen Markovnikov. An example is the hydrobromination of propylene to form 2-bromopropane. In it, hydrogen combines with a double bond with carbon, which is considered the most hydrogenated:
H3–CH = CH2 + HBr → CH3–BrCH – CH3.
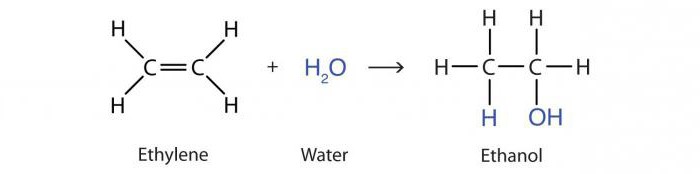
The reaction of the addition of water by alkenes under the action of acids is called hydration. The result is a propanol-2 alcohol molecule:
H3–HC = CH2 + H2O → CH3–OHCH – CH3.
When exposed to alkenes with sulfuric acid, the process of sulfonation occurs:
H3–HC = CH2 + HO − OSO − OH → CH3–H3CH – O − SO2−OH.
The reaction proceeds with the formation of acid esters, for example, isopropyl sulfuric acid.
Alkenes are subject to oxidation during their combustion under the action of oxygen with the formation of water and carbon dioxide gas:
2CH3–HC = CH2 + 9O2 → 6CO2 + 6H2ABOUT.
The interaction of olefinic compounds anddiluted potassium permanganate in the form of a solution leads to the formation of glycols or alcohols of diatomic structure. This reaction is also oxidative with the formation of ethylene glycol and bleaching solution:
3X2C = CH2 + 4H2O + 2KMnO4 → 3OHCH – CHOH + 2MnO2 + 2KOH.
Alkenic molecules can be involved in the polymerization process with a free radical or cation-anionic mechanism. In the first case, under the influence of peroxides, a polymer like polyethylene is obtained.
According to the second mechanism, cationic catalysts are acids, and organo-metallic substances are anionic with release of stereoselective polymer.
What are alkanes
They are also called paraffin or marginalacyclic hydrocarbons. They have a linear or branched structure, which contains only rich simple connections. All members of the homologous series of this class have the general formula CMr.X2n + 2.
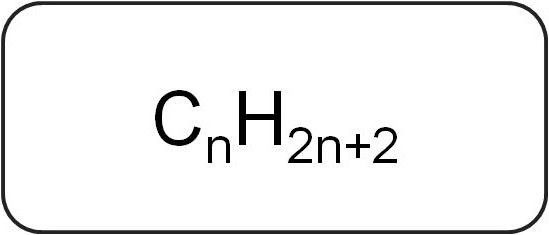
They contain only carbon and hydrogen atoms. The general formula of alkenes is formed from the designation of saturated hydrocarbons.
Names of alkanes and their characteristics
Самым простым представителем данного класса is methane. It is followed by substances like ethane, propane and butane. At the heart of their name is the root of the numeral in Greek, to which is added the suffix -an. Names of alkanes are listed in the IUPAC nomenclature.
The general formula of alkenes, alkynes, alkanes includesonly two kinds of atoms. These include elements of carbon and hydrogen. The number of carbon atoms in all three classes is the same, the difference is observed only in the number of hydrogen that can split off or join. Unsaturated compounds are obtained from saturated hydrocarbons. The representatives of paraffins in the molecule contains 2 hydrogen atoms more than olefins, which confirms the general formula of alkanes, alkenes. Alkenes are considered to be unsaturated due to the presence of a double bond.
If we compare the number of hydrogen and carbon atoms in alkanes, then the value will be maximum in comparison with other classes of hydrocarbons.
From methane to butane (from C1 to C4), substances exist in gaseous form.
Hydrocarbons of homologous gap from С are presented in liquid form.5 to C16. Starting with an alkane having 17 carbon atoms in the main chain, the physical state is transformed into a solid form.
Isomerism on the carbon skeleton and optical modifications of the molecule are characteristic of them.
Paraffin carbon valencies are consideredfully occupied by adjacent carbon or hydrogen to form a σ-type bond. From a chemical point of view, this causes their weak properties, which is why alkanes are called limit or saturated hydrocarbons, devoid of affinity.
They enter into substitution reactions associated with radical halogenation by sulfochlorination or nitration of the molecule.
Paraffins undergo an oxidation, burning or decomposition process at high temperatures. Under the action of reaction accelerators, the elimination of hydrogen atoms or the dehydrogenation of alkanes occurs.
What are alkynes?
They are also called acetylene hydrocarbons, in which a triple bond is present in the carbon chain. The structure of alkynes is described by the general formula CMr.X2n – 2. It shows that, unlike alkanes, acetylenic hydrocarbons lack four hydrogen atoms. They are replaced by a triple bond formed by two π-compounds.
Such a structure determines the chemical properties of this class. The structural formula of alkenes and alkynes clearly shows the unsaturation of their molecules, as well as the presence of double (H2C꞊CH2) and triple (HC≡CH) communication.
Name alkynes and their characteristics
The simplest representative is acetylene.or HC≡CH. He is also called ethin. It comes from the name of a saturated hydrocarbon, in which they remove the suffix -an and add -in. In the names of long alkynes, the number indicates the location of the triple bond.
Knowing the structure of saturated and hydrocarbonsunsaturated, it is possible to determine under which letter the general formula of alkynes is designated: a) CnH2n; c) CnH2n + 2; c) CnH2n-2; d) CnH2n-6. The correct answer is the third option.
Starting with acetylene and ending with butane (from C2 to C4), substances have a gaseous nature.

Hydrocarbons of homologous gap from C are in liquid form.5 to C17. Starting with an alkyne having 18 carbon atoms in the main chain, the physical state is transformed into a solid form.
They are characterized by isomerism on the carbon skeleton, on the position of the triple bond, and also on the interclass modifications of the molecule.
The chemical characteristics of acetylene hydrocarbons are similar to alkenes.
If alkynes have a triple bond, they arefunction as an acid to form alkynide salts, for example, NaC NaCNa. The presence of two π-bonds makes the molecule acetyledine sodium strong nucleophile, entering into substitution reactions.
Acetylene is chlorinated in the presence of copper chloride to produce dichloroacetylene, condensation under the action of halogenalkines with the release of diacetylene molecules.
Alkines are involved in addition reactions.electrophilic, the principle of which underlies halogenation, hydrohalogenation, hydrotation and carbonylation. However, such processes are weaker than those of alkenes with a double bond.
For acetylene hydrocarbons, addition reactions can occur based on the nucleophilic type of the alcohol molecule, primary amine, or hydrogen sulfide.