The atom is the minimal integral particle of matter.In its center is a nucleus, near which, like planets around the Sun, electrons rotate. Strange as it may seem, this smallest particle was discovered and formulated the concept of it still

Ancient scientists believed that the atom isultra-small pieces of any matter. The physical properties of the substance depend on their shape, massiveness, color and other parameters. For example, Democritus believed that the atoms of the fire are extremely sharp, because it burns, the particles of solids have rough surfaces that tightly adhere to each other, the water atoms are smooth and slippery, since they give the fluid fluidity.
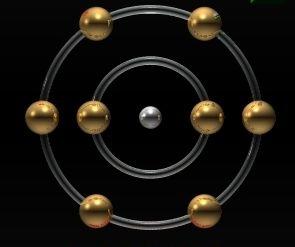
A more modern structure was proposed at the beginning20th century Japanese physicist Nagaoka. He presented the theoretical development, which is that the atom is a planetary system on a microscopic scale, and its structure is similar to the Saturn system. Such a structure proved to be erroneous. The model of the Bohr-Rutherford atom was closer to reality, but it failed to explain all the physical and electrical properties of corpuscles. Only the assumption that an atom is a structure that includes not only corpuscular properties but also quantum properties has been able to explain the largest number of observed realities.
Corpuscles can be in a connectedstate, and can - in the free. For example, an oxygen atom, in order to make up a molecule, connects with itself to another like particle. After an electric discharge, for example, a thunderstorm, it merges into

If the number of protons (elementary particlesnucleus) is analogous to the number of electrons rotating in orbits, then the atom is electrically neutral. If there is no identity, then the particle has a negative or positive discharge and is called an ion. As a rule, these charged particles are formed from atoms under the influence of electric fields, radiations of a different nature or high temperature. Ions are chemically hyperactive. These charged atoms are capable of dynamically reacting with other particles.







