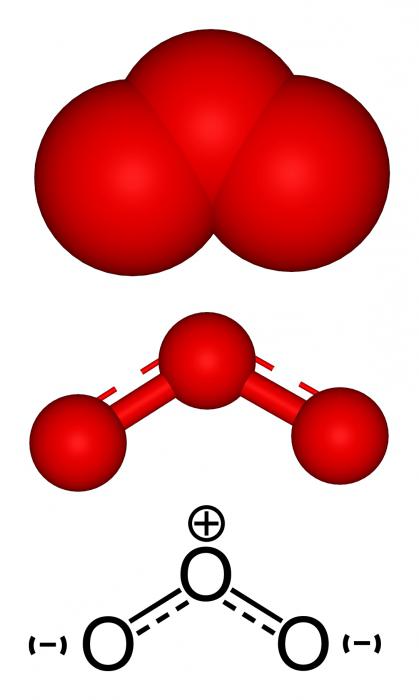
The most valuable for all mankind has such properties as ozone. The chemical element by which it is formed is oxygen O. In fact, ozone O3 - one of the allotropic modifications of oxygen,consisting of three formula units (О ÷ О ÷ О). The first and more known compound is oxygen itself, more precisely the gas, which is formed by its two atoms (O = O) -O2.

Allotropy is the ability of a single chemicalelement to form a number of different simple compounds on the basis of properties. Thanks to it, mankind has studied and uses such substances as diamond and graphite, monoclinic and rhombic sulfur, oxygen and ozone. A chemical element having such an ability is not necessarily limited to only two modifications, some have more.
Connection history
The constituent unit of many organic andmineral substances, including such as ozone - a chemical element, whose designation O-oxigen, in translation from Greek "oxys" - sour, and "gignomai" - to give birth.

For the first time a new allotropic modification of oxygenDuring experiments with electric discharges, the Dutchman Martin van Marun discovered in 1785, his attention was attracted by a specific smell. And a century later the Frenchman Shenbain noted the presence of the same after a thunderstorm, as a result of which the gas was called "smelling". But the scientists were somewhat deceived, considering that their sense of smell was sensed by ozone itself. The smell they felt belonged to organic compounds oxidized by interaction with O3, since the gas is very reactive.
Electronic structure
The same structural fragment has O2 and O3- chemical element. Ozone has a more complex structure. In oxygen, everything is simple - two oxygen atoms are connected by a double bond consisting of the ϭ and π-component, according to the valence of the element. ABOUT3 has several resonant structures.
Synthesis methods
To form a gas such as ozone, the chemical element oksigen must be in a gaseous medium in the form of individual atoms. Such conditions are created by the collision of oxygen molecules O2 with electrons during electrical discharges or other particles with high energy, and also when it is irradiated with ultraviolet light.
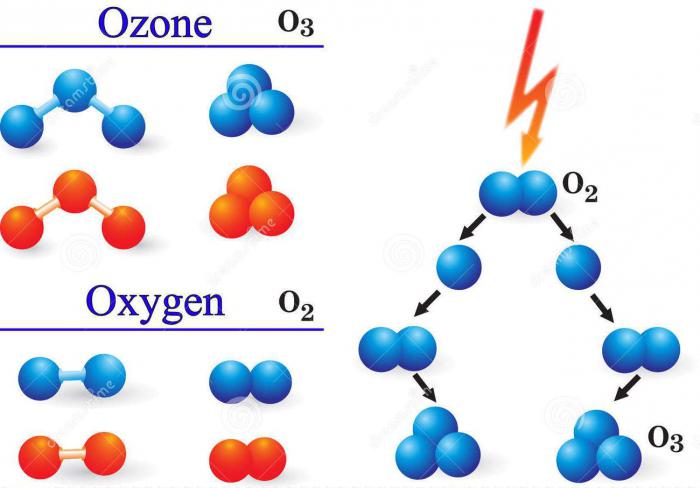
The lion's share of total ozone inThe natural conditions of the atmosphere are formed by the photochemical method. A person prefers to use other methods in chemical activity, such as, for example, electrolytic synthesis. It consists in placing platinum electrodes in the aqueous medium of the electrolyte and starting a current. Reaction Scheme:
H2O + O2 → About3 + H2 + e-
Physical properties
Oxygen (O) is a constituent unit of a substance such as ozone, a chemical element whose formula, as well as the relative molar mass, are indicated in the Mendeleyev table. Forming O3, the oxigen acquires properties that are fundamentally different from the properties of O2.

Gas of blue color - this is the usual state of suchcompound, like ozone. The chemical element, the formula, the quantitative characteristics - all this was determined in the identification and study of this substance. The boiling point for it is -111.9 ° C, the liquefied state has a dark violet color, while further lowering to -197.2 ° C, melting begins. In a solid aggregate state, ozone acquires a black color with a purple tint. Its solubility is ten times this oxygen property O2. At the most insignificant concentrations in the air, the smell of ozone is felt, it is sharp, specific and resembles the smell of metal.
Chemical properties
Very active, from a reactionary point of view,is the gas ozone. The chemical element that forms it is oxygen. The characteristics that determine the behavior of ozone in interaction with other substances are the high oxidizing ability and instability of the gas itself. At elevated temperatures it decomposes at an unprecedented rate, and the process accelerates catalysts such as metal oxides, chlorine, nitrogen dioxide and others. The properties of the oxidizer are inherent in ozone due to the peculiarities of the structure of the molecule and the mobility of one of the oxygen atoms, which, splitting off, turns the gas into oxygen: O3 → About2 + O
Oxygen (a brick from which molecules are builtsuch substances as oxygen and ozone) is a chemical element. As written in the reaction equations - O ·. Ozone oxidizes all metals, except gold, platinum and its subgroup. It reacts with gases in the atmosphere - oxides of sulfur, nitrogen and other. Organic substances do not remain inert, especially the rapid disruption of multiple bonds through the formation of intermediate compounds. It is extremely important that the reaction products are harmless to the environment and human. These are water, oxygen, higher oxides of various elements, carbon oxides. The interaction with ozone does not involve binary compounds of calcium, titanium and silicon with oxygen.

Application
The main area where "smelling" gas is usedIs ozonization. This method of sterilization is much more effective and safer for living organisms than disinfection with chlorine. When water is purified by ozone, there is no formation of toxic methane derivatives substituted with dangerous halogen.
Increasingly, such an ecological method of sterilizationfinds application in the food industry. Ozone treats refrigeration equipment, warehouses for products, with the help of it, eliminates odors.
For medicine, the disinfecting properties of ozone are also indispensable. They disinfect wounds, physiological solutions. Ozonize the venous blood, as well as "smelling" gas treat a number of chronic diseases.
Finding in nature and meaning
A simple ozone substance is an element of a gas compositionstratosphere, a region of near-Earth space located at a distance of about 20-30 km from the surface of the planet. Isolation of this compound occurs during the processes associated with electrical discharges, during welding, the operation of Xerox machines. But it is in the stratosphere that 99% of the total amount of ozone in the Earth's atmosphere is formed and contains.
The presence of gas innear-earth space. It forms the so-called ozone layer in it, which protects all life from the deadly ultraviolet radiation of the Sun. Strangely enough, but on a par with huge benefits, the gas itself is dangerous for people. Increasing the concentration of ozone in the air that a person breathes is harmful to the body, due to its extreme chemical activity.









