One of the most important places in scientific perceptionThe modern world is occupied by the so-called quantum theory. It is based on the proposition that the energy hidden in the electron can be calculated, since its magnitude can take only certain values. The most important consequence of this state of things is the conclusion that the state of an electron at one time or another can be described by a set of quantitative indicators - quantum numbers.
The most important in this theory is the mainquantum number. This term in modern physics is usually called a quantitative indicator, according to which a given state of an electron is referred to a certain energy level. The energy level, in turn, is a set of orbitals, the difference in energy value between them is extremely insignificant.
As follows from this provision, the main thingthe quantum number can be equal to one of the positive natural numbers. In this case, another fact is of fundamental importance. After all, in the case of the transition of an electron to a different energy level, the main quantum number will necessarily change its value. It is quite appropriate to draw a parallel with Niels Bohr's model, where an elementary particle passes from one orbit to another, as a result of which a certain amount of energy is released or absorbed.

Главное квантовое число самым непосредственным is related to the orbital quantum number. The thing is that any energy level is heterogeneous in nature and includes several orbitals. Those of them that have the same energy value form a separate sublevel. To find out to which sublevel one or another orbital belongs, and use the concept of "orbital quantum number." To calculate it, it is necessary to take the unit from the principal quantum number. Then all natural numbers from zero to this exponent u will be the orbital quantum number.
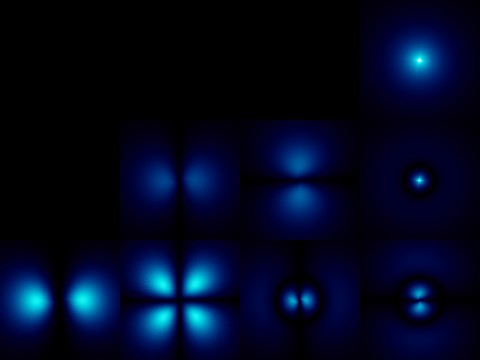
The most important function of this quantitativecharacteristic is that with its help, not only does an electron correlate with a particular sublevel, but also the trajectory of the motion of a given elementary particle is characterized. Hence, by the way, and the letter designation of orbitals, which are known even from the school course of chemistry: s, d, p, g, f.

Another important characteristic of the situationelectron is the magnetic quantum number. Its basic physical meaning is that it is possible to characterize the projection of the angular momentum with respect to the direction coinciding with the direction of the magnetic field. In other words, it is necessary in order to distinguish electrons that occupy orbitals, the quantum number of which is the same.
The magnetic quantum number can vary inlimits 2l + 1, where l is the quantitative characteristic of the orbital quantum number. In addition, a magnetic spin number is also extracted, which is necessary in order to characterize the quantum property of an elementary particle in its pure form. Spin is nothing more than a moment of momentum that can be compared to the rotation of an electron around its own imaginary axis.