Nowadays, batteries are the mostcommon power sources for electronics and small appliances. The need to replace them occurs quite often. In order to make the best choice when buying a new electroplating cell, you should pay attention not only to the size of the batteries and the name of the manufacturer. This article will find answers to the following questions: what form are these power sources? What are the types of batteries in size? How are galvanic cells labeled and what should be paid attention to when buying so that the power source lasts a long time?
Types of batteries
The classification of batteries is carried out depending on the materials from which their active components are made: the anode, cathode and electrolyte.
There are five types of modern food sources:
- saline,
- alkaline,
- mercury,
- silver,
- lithium.
Battery types by size will be listed below. And now let's take a closer look at each of the indicated classes of galvanic cells.
Salt batteries
Salt batteries were created in the second halftwentieth century. They replaced the previously existing manganese-zinc power sources. The size of the batteries has not changed, but the technology of manufacturing these electroplating cells has become different. In salt power sources, ammonium chloride solution is used as the electrolyte. It contains electrodes made of zinc and manganese oxide. The connection between the individual electrolytes is carried out using a salt bridge.
The main advantage of such batteries is their low cost. These galvanic batteries are the cheapest among all existing ones.

Disadvantages of salt batteries:
- during the discharge period the voltage decreases significantly;
- shelf life is small and is only 2 years;
- by the end of the guaranteed storage period, the capacity is reduced by 30-40 percent;
- at low temperature, the capacity decreases to almost zero.
Alkaline batteries
Such batteries were invented in 1964. Another name for these food sources is alkaline (from the English word alkaline, which literally means “alkaline”).
The electrodes of such batteries are made of zinc and manganese dioxide. The electrolyte is alkali potassium hydroxide.
Today, these batteries are the most common, because they are great for most electronic devices.
The advantages of alkaline power sources:
- have a greater capacity in comparison with salt and, as a result, a longer service life;
- can operate at low ambient temperatures;
- possess the improved tightness, that is the probability of a leakage is reduced;
- have a longer shelf life, which is 5 years;
- possess the reduced self-discharge rate in comparison with salt batteries.

Disadvantages of alkaline power sources:
- the discharge period is characterized by a gradual decrease in the output voltage;
- Alkaline batteries are similar in size to salt, but the cost and mass of alkaline power sources are higher.
Mercury batteries
In such a battery, the anode is made of zinc,the cathode is of mercury oxide. The electrodes are separated by a separator and a diaphragm, which is impregnated with 40% potassium hydroxide solution. Alkali is used here as electrolyte. Thanks to this composition, this power source can work as a battery. But with cyclic operation, the galvanic cell degrades, its capacity decreases.
Merits of mercury batteries:
- stable voltage;
- high performance capacity and energy density;
- the ability to work both at high and at low ambient temperatures;
- Long shelf life, which is 10 years.
Disadvantages of mercury power sources:
- high price;
- possibility of dangerous exposure to mercury vapor in the event of depressurization;
- the need to establish a process of collection and disposal.
Silver batteries
In the silver battery for the production of the anode is used zinc, for the cathode - silver oxide. The electrolyte is sodium or potassium hydroxide.

This category includes batteries for watches, the dimensions of which will be given below. The advantages of silver power sources are the following:
- voltage stability;
- the presence of high capacity and energy density;
- insensitivity to ambient temperature;
- Long service life and storage.
The disadvantage of such batteries is their high cost.
Lithium batteries
In such a battery, the cathode is made of lithium. It is separated from the anode with a separator and a diaphragm, which is impregnated with organic electrolyte.
Advantages of lithium batteries:
- constant pressure;
- high capacity and energy density;
- independence of energy intensity from load current;
- small weight;
- long shelf life of up to 12 years;
- immunity to temperature extremes.

The disadvantages of lithium batteries can be attributed only to their high cost.
As stated above, power supplies have differentchemical composition. Also significantly differ from each other in the shape and size of batteries. Galvanic cells have different heights, diameters and voltages. Consider the classification of batteries in accordance with these parameters.
Battery classification by size
Depending on the voltage, height, diameter andforms, power sources can be systematized in a certain way. One of the most popular classification systems is the American. It is presented in the figure below. Such standardization is convenient, it is used in many countries.
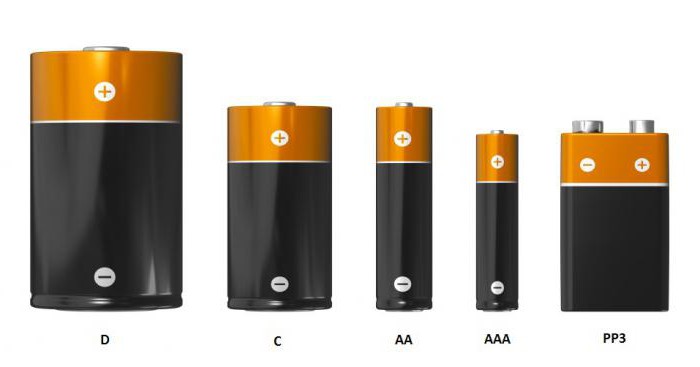
According to the American system, power supplies are classified as follows:
Name | Height, mm | Diameter, mm | Voltage, V |
D | 61,5 | 34,2 | 1,5 |
|---|---|---|---|
FROM | 50,0 | 26,2 | 1,5 |
AA | 50,5 | 14,5 | 1,5 |
AAA | 44,5 | 10,5 | 1,5 |
PP3 | 48,5 | 26,5 | 9,0 |
In addition to the class indicated in the table, sourcesfood have an everyday name, which is used by the people. For example, the size of an AA battery is comparable to the size of a human finger, therefore the “popular” name of this galvanic cell is a “finger” battery, or “two A”. But the C power source is referred to in everyday life as "inch". A galvanic cell D is called a “barrel.” And an AAA battery, the dimensions of which are similar to the parameters of the smallest finger of a person, is not for nothing called the “little finger”, or “three A”. The PP3 power source is called the crown.
Also widely used in electronicsminiature round batteries, the sizes and names of which are diverse. More detailed information on silvery “pills” and the classification of such power supplies are given below.
Batteries "pills": sizes and names
Another name for a miniature round battery isdry item. Such power sources consist of an anode made of silver oxide, a zinc cathode and an electrolyte. The latter is a mixture of salts, which has a pasty consistency.
Разные производители нередко присваивают таким power sources designations that differ from standard ones. Below is a classification table that lists the alternative names and sizes of watch batteries.

It is these miniature silver "pills"forced to work mechanisms of modern watches. When it comes time to replace the battery, you may be confronted with the question of what kind of power source is suitable in this situation? For example, if the element used in the watch is 399, you can put a miniature battery instead, which, depending on the manufacturer, may be named V399, D399, LR57, LR57SW, LR927, LR927SW or L927E. Under these names will be made "tablet", whose height is 2.6 millimeters and a diameter of 9.5.
Battery size is not the only parameterto which you should pay attention when buying power supplies. In order to learn how to decipher information, which is located on the galvanic elements, you need to familiarize yourself with the basic principles of their labeling.
Battery labeling
International Electrotechnical Commission (IEC)created a certain system of designations, according to which all batteries should be labeled. On the case of the power source must be indicated information about its energy intensity, composition, size, class and voltage value. On the example of the battery shown below, we consider in more detail all the elements of labeling.

The information on the power supply indicates the following:
- electric charge of the electroplating element is 15 Ah;
- power source class - AA, that is, it is a “finger-type” battery;
- voltage is 1.5 volts.
And what does the inscription "LR6" mean? This, in fact, is the label, which gives information about the chemical composition and class of the power source. Types of batteries have the following letter designations:
- salt - R;
- alkaline - LR;
- silver - SR;
- lithium - CR.
Classes of batteries are indicated by these numbers:
- D - 20;
- C - 14;
- AA - 6;
- AAA - 03;
- PP3 - 6/22.
Now you can decipher the LR6 marking ongiven figure. The letters here indicate that this is an alkaline galvanic cell, and the number indicates the size of a “finger-type” battery, that is, it indicates that the power source belongs to class AA.
Scope of application and features of battery selection
First of all it should be noted that allgalvanic cells meet the requirements of unification, that is, the consumer can easily replace the power source of one manufacturer with a similar battery of another. There is only one caveat: do not use current sources made by different companies or all the more to different types in one device. This will significantly reduce battery life.
When choosing power sources need to payattention to packaging. Often, the manufacturer indicates on it the devices in which it is recommended to use these particular batteries. If this information is not provided, the tips below will help you make the right choice.
Salt batteries have low capacity in0.6-0.8 A * h and are used in devices with low power consumption. It can be remote controls, electronic thermometers, testers, floor or kitchen scales. Salt cells can also be used as watch batteries. The sizes of such current sources are similar to the corresponding parameters of alkaline ones, however, their areas of application vary significantly. After all, if you use salt batteries in devices with an electric motor, flashlights or cameras, then their lifespan can be only 20-30 minutes. Such galvanic cells are not designed for heavy loads.
Щелочные батарейки обладают достаточно большой with a capacity of 1.5-3.2 Ah This allows you to successfully use them in devices that have high power consumption. These devices include digital cameras with flash, flashlights, children's toys, office phones, computer mice, etc. Batteries designed specifically for cameras give off energy faster. This has a positive effect on the speed of the cameras. If you use the alkaline power source in devices with low power consumption, the batteries will show excellent results, their service life will be several years.

Twenty - thirty years ago mercury batteriesThey were widely used in devices such as electronic clocks, pacemakers, hearing aids, and military equipment. To date, the use of these food sources is limited. In many countries, it is forbidden to produce and operate such galvanic cells due to the fact that mercury is a toxic substance. In the case of the use of these current sources, it is necessary to organize their separate collection and disposal in accordance with safety requirements.
Silver batteries did not receive massspread due to the high cost of the metal. However, miniature power sources of this type are widely used in wristwatches, motherboards for laptops and computers, hearing aids, music cards, key rings and other devices where it is impossible to use larger batteries.
Lithium batteries have a longer life.services in comparison even with the best alkaline. Therefore, these power sources are used in devices that have high power consumption. This may be computer and photographic equipment, medical equipment.
Conclusion
A battery is a product that, despite itssmall sizes can be dangerous. You can not disassemble the power source, throw it into the fire and, of course, try to recharge. Online you can find tips on how to give a battery a second life. Do not try to conduct such experiments, because it can be dangerous.
When buying new batteries should be paidattention not only to the manufacturer and suitable dimensions, but also to the chemical composition of power sources. For this you need to be able to read the markings. Properly selected batteries will serve for a long time and efficiently.








