The events of the physical world are inseparably connected withtemperature changes. Every person gets acquainted with it in early childhood, when he realizes that the ice is cold and that it boils hot water. At the same time, there comes an understanding that temperature change processes do not occur instantaneously. Already later at school a student studies that this is connected with thermal movement. And the processes associated with temperature, highlighted a whole section of physics.

What is temperature?
This scientific concept is introduced to replace the ordinaryterms. Words like hot, cold or warm constantly appear in everyday life. They all talk about the degree of body heat. That is how it is defined in physics, only with the addition that it is a scalar quantity. After all, temperature has no direction, but only a numerical value.
In the international system of units (SI) temperaturemeasured in degrees Celsius (ºС). But in many formulas describing thermal phenomena, it is required to translate it into Kelvin (K). There is a simple formula for this: T = t + 273. In it T is the temperature in Kelvin, and t is in Celsius. The concept of absolute zero temperatures is associated with the Kelvin scale.
There are several more temperature scales.In Europe and America, for example, in the course of Fahrenheit (F). Therefore, they must be able to record in Celsius. To do this, it is necessary to subtract 32 from the testimony in F, then divide it by 1.8.

Home experiment
In his explanation you need to know such things as temperature, thermal movement. Yes, and perform this experience is simple.
It will need to take three tanks.They should be large enough to easily fit the hands. Fill them with water of different temperatures. In the first, it should be very cold. In the second - heated. In the third to pour hot water, such in which the hand will be possible to hold.
Now the experience itself. Immerse your left hand in a container of cold water, right - with the hottest. Wait a couple of minutes. Remove them and immediately immerse in a vessel with warm water.
The result will be unexpected.The left hand will feel that the water is warm, the right will have a sensation of cold water. This is due to the fact that at first thermal equilibrium is established with those liquids in which the hands are immersed initially. And then this balance is sharply disturbed.
The main provisions of the molecular kinetic theory
She describes all thermal phenomena. And these statements are quite simple. Therefore, in talking about thermal movement, these provisions need to be known.
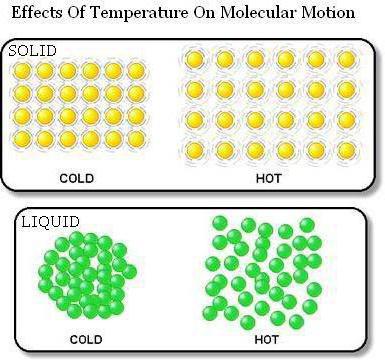
First:substances are formed by tiny particles located at some distance from each other. Moreover, these particles can be both molecules and atoms. And the distance between them is many times greater than the size of the particles.
Second: in all substances there is a thermal movement of molecules, which never stops. Particles while moving randomly (randomly).
Third: the particles interact with each other. This action is due to the forces of attraction and repulsion. Their value depends on the distance between the particles.
Confirmation of the first position of the ICB
The proof that the bodies are made up of particlesbetween which there are gaps, serves their thermal expansion. So, when the body is heated, its size increases. This happens because of the removal of particles from each other.
Another confirmation of this isdiffusion. That is, the penetration of molecules of one substance between the particles of another. Moreover, this movement is mutual. Diffusion is the faster, the further away from each other molecules are located. Therefore, in gases, mutual penetration will occur much faster than in liquids. And in solids, diffusion takes years.
By the way, the latter process also explains the thermal movement. After all, the mutual penetration of substances into each other occurs without any intervention from the outside. But it can be accelerated if the body is heated.
Confirmation of the second ICB provision
Vivid proof that there isthermal motion is the Brownian motion of particles. It is considered for suspended particles, that is, for those that are substantially larger than the substance molecules. These particles can be dust particles or grains. And it is necessary to place them in water or gas.
The reason for the random motion is weighted.the particles are that molecules act on it from all sides. Their action is random. The magnitude of the impacts at each time point is different. Therefore, the resultant force is directed one way or the other.
If we talk about the speed of thermal motion of molecules, then there is a special name for it - the quadratic mean. It can be calculated by the formula:
v = √ [(3kT) / m0n.
In it T is the temperature in Kelvin, m0 Is the mass of one molecule, k is the Boltzmann constant (k = 1.38 * 10-23 J / C).

Confirmation of the third ICB provision
Particles attract and repel. In explaining the many processes associated with thermal motion, this knowledge is important.
After all, the interaction forces depend on the aggregatestates of matter. So, the gases are practically nonexistent, since the particles are removed so strongly that their action does not manifest itself. In liquids and solids, they are tangible and ensure the preservation of the volume of a substance. In the latter, they also guarantee the maintenance of form.
Доказательством существования сил притяжения и repulsion is the appearance of elastic forces during deformation of bodies. So, when lengthening, the forces of attraction between molecules are enhanced, and during compression - repulsion. But in both cases, they return the body to its original shape.

Average energy of thermal movement
It can be written from the main MKT equation:
(pV) / N = (2E) / 3.
In this formula, p is the pressure, V is the volume, N is the number of molecules, E is the average kinetic energy.
On the other hand, this equation can be written as:
(pV) / N = kT.
If you combine them, you get the following equality:
(2E) / 3 = kT.
It follows this formula for the average kinetic energy of molecules:
E = (3kT) / 2.
This shows that energy is proportionaltemperature of matter. That is, as the latter rises, the particles move faster. This is the essence of the thermal motion, which exists as long as there is a temperature different from absolute zero.








