Binary compounds are substances that are formed by two different chemical elements. This term is used to describe the qualitative and quantitative composition of inorganic compounds.
Binary chemical compounds are considered importantobject in the study of the nature of substances. When describing them, the following concepts are used: bond polarization, oxidation degree, valence. These chemical terms make it possible to understand the essence of the formation of a chemical bond, the structural features of inorganic substances.
Let us consider the main classes of binary compounds, the features of their chemical structure and properties, and some areas of their industrial application.
Oxides
This class of inorganic substances is the most common in nature. Among the known representatives of this group of compounds, we distinguish:
- silicon oxide (river sand);
- hydrogen oxide (water);
- carbon dioxide;
- clay (aluminum oxide);
- iron oxides (iron oxides).
Such binary compounds are complex substances in which oxygen is necessarily present, exhibiting an oxidation state of -2.
Aggregate state of oxides
Compounds of copper, calcium, iron arecrystalline solids. The same aggregate state has oxides of some non-metals, for example hexavalent sulfur, pentavalent phosphorus, silicon. The liquid under normal conditions is water. The vast majority of oxygen compounds of nonmetals are gases.
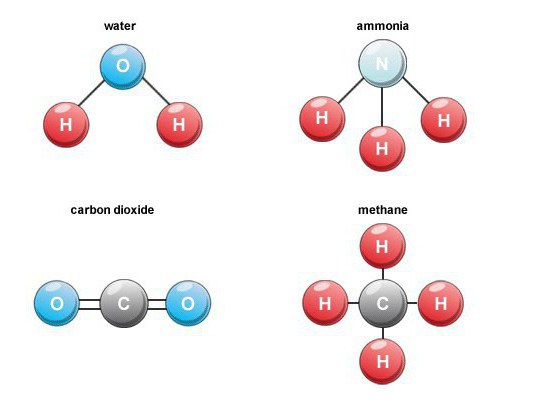
Features of education
Many binary oxygen compounds are formedin nature. For example, when burning fuel, breathing, decaying organic substances, carbon dioxide (carbon monoxide 4) is formed. In the air, its volume content is about 0.03 percent.
Similar binary compounds are productsactivity of volcanoes, as well as a constituent part of mineral water. Carbon dioxide does not support combustion, so this chemical compound is used to extinguish fires.
Volatile hydrogen compounds
Such binary connections are an important groupsubstances in the composition of which hydrogen is present. Among representatives of industrial importance, we note methane, water, hydrogen sulphide, ammonia, and also halogenated hydrogen.
Part of the volatile hydrogen compounds is present in soil waters, living organisms, so we can talk about their geochemical and biochemical role.
To make binary compounds of this kind, hydrogen, having a valence, comes first. The second element is a nonmetal having a negative oxidation state.
To arrange indices in a binary connectionthe least common multiple is determined between the valences. The number of atoms of each element is determined by dividing it by the valence of each element that is part of the compound.

Hydrogen chloride
Consider the formulas of binary compounds:hydrogen chloride and ammonia. It is these substances that are important for the modern chemical industry. HCl under normal conditions is a gaseous compound, highly soluble in water. After dissolving the hydrogen chloride gas, hydrochloric acid is formed, which is used in many chemical processes and production chains.
This binary compound is contained in the gastric juice of humans and animals, is a barrier to pathogens that penetrate with food into the stomach.
Among the main fields of application of hydrochloric acid, we distinguish the production of chlorides, the synthesis of chlorine-containing products, etching of metals, the purification of pipes from oxides and carbonates, leather production.
Ammonia having the formula NH3, is a colorless gas havingspecific sharp odor. Its unlimited solubility in water makes it possible to obtain ammonia alcohol, claimed in medicine. In nature, this binary compound is formed during the decay of organic products, in which nitrogen is present.

Classification of oxides
The oxygen-containing binary compound of a metal having a valence of 1 or 2 is the basic oxide. For example, this group includes oxides of alkali and alkaline-earth metals.
Oxides of nonmetals, as well as metals with a valence greater than 4, are acidic compounds.
Depending on the chemical properties of representatives of this class are divided into salt-forming and non-salt-forming groups.
Among typical representatives of the second group, mention carbon monoxide (CO), nitrogen oxide 1 (NO).
Forming systematic names of compounds
Среди заданий, предлагаемых выпускникам, сдающим state examination in chemistry, there is this: "Make molecular formulas for possible binary oxygen compounds of sulfur (nitrogen, phosphorus)." In order to cope with the task, it is necessary to have an idea not only about the algorithm, but also about the features of the nomenclature of this class of inorganic substances.
When forming the name of a binaryconnections, initially indicate the element that in the formula is located on the right, adding the suffix "id". Next, specify the name of the first element. For covalent compounds, attachments are added, along which a quantitative relationship can be established between the constituents of the binary compound.
For example, SO3 - sulfur trioxide, N2ABOUT4 - diazotide tetroxide, I2SL6 - hexachloride of the diode.
If a chemical element capable of exhibiting different degrees of oxidation is present in the binary compound, the state of oxidation is indicated in parentheses after the name of the compound.
For example, two compounds of iron differ in name: FeCL3 - iron oxide (3), FeCL2 - iron oxide (2).
For hydrides, in particular non-metallic elements, use trivial names. So, H2O - water, HCL - hydrogen chloride, HI - hydrogen iodide, HF - hydrofluoric acid.

Cations
Positive ions of those elements thatable to form only one stable ion, give the same names as the characters themselves. These include all representatives of the first and second groups of the periodic system of Mendeleev.
For example, sodium and magnesium cations have the form: Na+, Mg2+. Transitional elements are able to form several types of cations, therefore, in the name, it is necessary to indicate the valence, which is manifested in each individual case.
Anions
For simple (monatomic) and complex (polyatomic) anions, the suffix -id is used.
Common oxo anion definedelement is the suffix –s. For the oxoanion element, in the formula with a lower degree of oxidation, the suffix -it is used. For the minimum oxidation state, the hypo prefix is used, and for the maximum value, the first is. For example, ion O2- is an oxide ion, and O- - peroxide.
There are various trivial names of hydrides. For example, N2X4 called hydrazine, and PH3 called phosphine.
Sulfur-containing oxo anions have the following names:
- CO42- - sulfate;
- FROM2ABOUT32- - thiosulfate;
- NCS- - Thiocyanate.
Salt
Many final chemistry tests offerthe next task: "Compose the formulas of binary metal compounds". If such compounds contain anions of chlorine, bromine, and iodine, such compounds are called halides and belong to the class of salts. In formulating the formulas of these binary compounds, the metal is put first, then the corresponding halide ion.
To determine the number of atoms of each element, the smallest multiple between the valences is found, while dividing indices are obtained.
Such compounds have a high melting point.and boiling, good solubility in water, under normal conditions, they are solids. For example, sodium and potassium chlorides are part of seawater.
Salt people use since ancient times.Currently, the use of this binary compound is not limited to the use in food. The electrolysis of an aqueous solution of sodium chloride produces metallic sodium and chlorine gas. These products are used in various industrial processes, for example, for the production of sodium hydroxide, hydrogen chloride.
The value of binary compounds
This group includes a huge amountsubstances, therefore it is possible to speak with confidence about the scale of their use in different spheres of human activity. Ammonia in the chemical industry is used as a precursor in the manufacture of nitric acid, the production of mineral fertilizers. It is this binary compound used in fine organic synthesis that has been used for a long time in refrigeration units.
Due to the unique hardness of tungsten carbide,This compound has been used in the manufacture of various cutting tools. The chemical inertness of this binary compound allows its use in aggressive environments: laboratory equipment, furnaces.
"Laughing gas" (nitric oxide 1) mixed with oxygen is used in medicine for general anesthesia.
All binary compounds have a covalent or ionic nature of the chemical bond, molecular, ionic or atomic crystal lattice.

Conclusion
При составлении формул бинарных соединений It is necessary to observe a certain sequence of actions. First, an element is recorded that exhibits a positive degree of oxidation (has a smaller value of electrical negativity). When determining the value of the degree of oxidation of the second element of the eight, subtract the number of the group in which it is located. If the numbers obtained differ from each other, the smallest common multiple is determined, then the indices are calculated.
In addition to oxides, these compounds are rankedcarbides, silicides, peroxides, hydrides. Aluminum carbides and calcium are used for laboratory production of methane and acetylene, peroxides are used in the chemical industry as strong oxidizing agents.
Такой галогенид, как фтороводород (плавиковая acid), is used in electrical engineering when soldering. Among the most important binary compounds, without which it is difficult to imagine the existence of living organisms, water leads. The structural features of this inorganic compound are studied in detail in the school chemistry course. It is by her example that the guys get an idea of the sequence of actions when drawing up formulas for binary compounds.
In conclusion, we note that it is difficult to find such a sphere of modern industry, an area of human life, wherever various binary compounds are used.