A plain bottle of food vinegar that you canfound in the kitchen of any housewife, has in the composition of many other acids and vitamins. Adding a couple drops of product to cooked food, salads causes a natural flavor enhancement. But few of us seriously thought about the properties and real scales of using the main component. - acetic acid.
What is this substance?
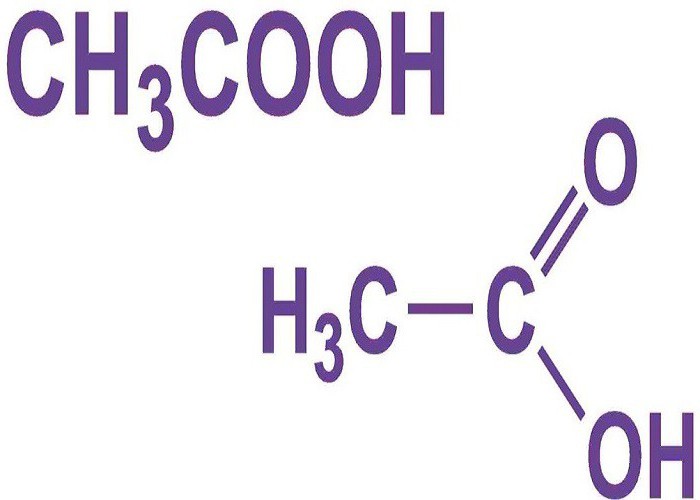
Acetic acid formula CH3СООН, что относит ее к ряду жирных карбоновых acids. The presence of one carboxyl group (COOH) refers it to monobasic acids. The substance is found on the globe in an organic form and is obtained synthetically in laboratories. Acid is the simplest, but no less important representative of its series. Easily soluble in water, hygroscopic.
Physical properties of acetic acid and density vary depending on temperature. At room temperature at 20aboutC acid is in a liquid state, has a density of 1.05 g / cm3. It has a peculiar smell and sour taste. The solution of the substance without impurities hardens and goes into crystals at temperatures below 17aboutC. The boiling process of acetic acid begins at temperatures above 117aboutC. Methyl group (CH3The formulas of acetic acid are obtained by the interaction of alcohols with oxygen: fermentation of alcoholic substances and carbohydrates, souring of wines.
A bit of history
Открытие уксуса было одним из первых в ряду acids and committed in stages. At first, 8th century Arab scientists began to extract acetic acid by distillation. However, in ancient Rome, this substance obtained from sour wine, was used as a universal sauce. The name itself from ancient Greek translates as "sour." In the 17th century, scientists in Europe managed to extract the pure substance of a substance. At that time, they derived a formula and discovered an unusual ability - acetic acid in the vapor state was ignited by a blue fire.
Up until the 19th century, scientists found a presenceacetic acid in organic form only - as part of the compounds of salts and esters. In the composition of plants and their fruits: apples, grapes. In humans and animals: sweat, bile. At the beginning of the 20th century, Russian scientists randomly extracted acetaldehyde from the reaction of acetylene with mercuric oxide. At present, the consumption of acetic acid is so great that its main production occurs only on a synthetic basis on a huge scale.
Methods of extraction
Will acetic acid be pure or with impurities in solution зависит от метода добычи.Food grade acetic acid is obtained by the biochemical method in the process of ethanol fermentation. In industry, several methods of acid extraction are distinguished. As a rule, reactions are accompanied by high temperature and the presence of catalysts:
- Methanol in reaction with carbon (carbonylation).
- Oxidation of the oil fraction by oxygen.
- Pyrolysis of wood.
- Oxidation of acetaldehyde by oxygen.

Industrial way more efficient and more economicalbiochemical. Thanks to the industrial method, the production of acetic acid in the 20th and 21st centuries has grown hundreds of times, compared with the 19th century. Today, the synthesis of acetic acid by carbonylation of methanol gives more than 50% of the total volume produced.
Physical properties of acetic acid and its effect on the indicator
In the liquid state, acetic acid is colorless.An acidity level of pH 2.4 is easily checked by litmus test. Acetic acid, when it hits the indicator, turns it red. The physical properties of acetic acid change visually. When the temperature drops below 16aboutC, the substance takes a solid form and resemblessmall crystals of ice. It is easily soluble in water and interacts with a wide range of solvents, in addition to hydrogen sulfide. Acetic acid reduces the total volume of liquid when diluted with water. Independently describe the physical properties of acetic acid, its color and consistency, which you see in the following image.

The substance is flammable at temperatures from 455aboutC with a heat release of 876 kJ / mol.The molar mass is 60.05 g / mol. The physical properties of acetic acid as an electrolyte in reactions are weak. The dielectric constant is 6.15 at room temperature. Pressure, like density, - variable physical properties of acetic acid. At a pressure of 40 mm. Hg. Art. and temperature 42aboutThe boiling process will begin. But already at a pressure of 100 mm. Hg. Art. boiling will occur only at 62aboutFROM.
Chemical properties
Reacting with metals and oxides,the substance exhibits its acidic properties. Perfectly dissolving more complex compounds, the acid forms salts called acetates: magnesium, lead, potassium, etc. The pK value of the acid is 4.75.

При взаимодействии с газами уксус вступает в substitution reaction with subsequent displacement and the formation of more complex acids: chloroacetic, iodoacetic. Dissolving in water, the acid dissociates with the release of acetate ions and hydrogen protons. The degree of dissociation is 0.4 percent.
Physical and chemical properties of acetic moleculesacids in crystalline form create diameters on hydrogen bonds. Also, its properties are necessary when creating more complex fatty acids, steroids and sterol biosynthesis.
Laboratory tests
Detect acetic acid in solution can be forby identifying its physical properties, such as odor. It is enough to add a stronger acid to the solution, which will begin to displace vinegar salts with the release of its vapor. Laboratory distillation of CH3COONa and H2CO4 it is possible to obtain acetic acid in dry form.
Let's make an experiment from the school program in chemistry 8class. The physical properties of acetic acid are clearly demonstrated by the chemical dissolution reaction. It is enough to add copper oxide to the substance and slightly heat it. The oxide is completely dissolved, making a bluish solution.
Derivatives
Qualitative reactions of a substance with manysolutions form: esters, amides and salts. However, during the production of other substances, the requirements for the physical properties of acetic acid remain high. It should always have a high degree of dissolution, which means that it does not have external impurities.
В зависимости от концентрации уксусной кислоты aqueous solution emit a number of its derivatives. A concentration of more than 96% is called glacial acetic acid. Acetic acid in 70-80% can be purchased at grocery stores, where it will be called - vinegar essence. Table vinegar has a concentration of 3-9%.

Acetic acid and daily life
In addition to nutritional features, acetic acidpossesses a number of physical properties, which mankind has found its application in everyday life. A solution of a low concentration substance easily removes plaque from metal products, the surface of mirrors and windows. The ability to absorb moisture is also beneficial. Vinegar well removes odors in musty rooms, removes stains from vegetables and fruits on clothes.
As it turned out, the physical property of acetic acid - remove grease from the surface - can findapplication in traditional medicine and cosmetology. A weak solution of food vinegar is processed on the hair to give it shine. The substance is widely used for the treatment of colds, the removal of warts and skin fungi. The use of vinegar as part of cosmetic wraps to combat cellulite is gaining momentum.
Use in production
In the compounds of salts and other complex substances, acetic acid acts as an indispensable element:
- The pharmaceutical industry. To create: aspirin, antiseptic and antibacterial ointments, phenacetin.
- Synthetic fiber production. Non-combustible films, cellulose acetate.
- Food industry. For the successful preservation, preparation of marinades and sauces, as a food supplement E260.
- Textile industry. Included in dyes.
- Manufacture of cosmetics and hygiene products. Aromatic oils, creams to improve skin tone.
- The manufacture of mordants. Used as an insecticide and weed control.
- Production of varnishes. Technical solvents, acetone production.

Acetic acid production annuallyincreases. Today, its volume in the world is more than 400 thousand tons per month. Acid is transported in durable steel tanks. Storage in plastic containers in many industries due to the high physical and chemical activity of acetic acid is prohibited or limited to a period of several months.
Security
High concentration acetic acid hasthird degree of ignition and emits toxic fumes. It is recommended to wear special gas masks and other personal protective equipment when working with acid. The lethal dose for the human body is from 20 ml. At the moment of ingestion of the substance inside, the acid firstly burns the mucous membrane, and then affects the remaining organs. In such cases, immediate hospitalization is needed.
After acid has come into contact with exposed skin, rinse immediately with running water. A superficial burn with acid can cause tissue necrosis, which also requires hospitalization.
Interesting Facts
Physiologists have found that man doesn’tAcetic acid is required - food additives can be dispensed with. But for people with acid intolerance, as well as stomach problems, the substance is contraindicated.

Acetic acid is used in typography.
The substance was found in small quantities in honey, bananas and wheat.
After cooling the acetic acid and shaking the container sharply with it, you can observe its sharp hardening.
A small concentration of acetic acid can reduce the pain symptom of an insect bite, as well as minor burns.
Eating foods with a low content of acetic acid lowers cholesterol in the body. The substance stabilizes sugar levels in diabetics well.
The use of protein and carbohydrate foods, along with a small amount of acetic acid, increases their digestibility by the body.
If the food is too salted, just add a few drops of vinegar to smooth out the saltiness.
At last
Millennia of use of acetic acidled to the fact that its physical and chemical properties find their application at every step. Hundreds of possible reactions, thousands of useful substances, thanks to which mankind goes further. The main thing is to know all the features of acetic acid, its positive and negative qualities.
Do not forget about the benefits, but always needremember what harm can be caused by careless handling of high concentration acetic acid. In its danger, it stands next to hydrochloric and sulfuric acid. Always be mindful of safety when using acid. Dilute the essence correctly and carefully with water.






